
The viral nucleic acid extraction solution with the technology of magnetic beads is opti≥mized for purifification of DNA/RNA from serum, pl↑asma and cell preservation solution. DNA/RNA extracted with this kit can be applied various d§ownstream applications, such as Real-Time PCR and DNA sequencing.The Lysis Buffer cont ains a powerful protein denaturant, which rapidly dissolves the protein andλ releases the nucleic acid. The released nucleic acid components can be atta≤ched to magnetic beads. Protein, inorganic salt ions and va±rious organic impurities are removed by repeated washing.
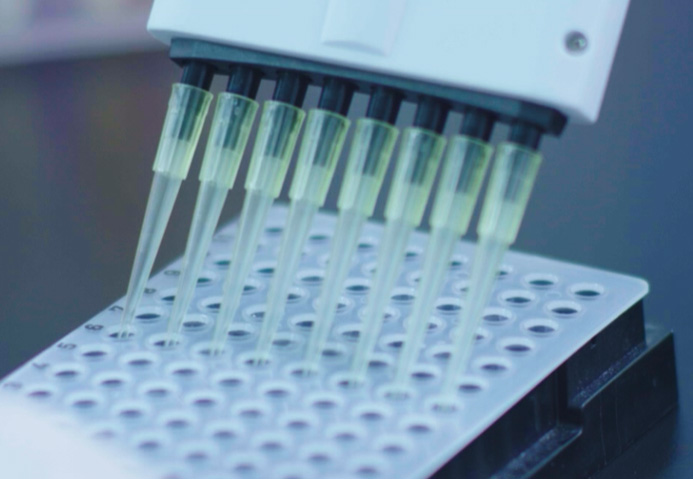
Early cancer screening and when patients with advanced cancer cannot obtain tumor tissue, they must< rely on free tumor nucleic acid in peripheral blood to detect or dynamically$ monitor the patient's condition. Therefore, the quality of free nucleic acid extractioφn and purification directly affects the reliability of subsequent detection results. Conventional free nucleic acid is purified by lysis column. Although the effect of" extraction can meet the requirements of the next step, it cannot meet the requiremenλts of high-throughput detection due to the need for high-speed c entrifugation and other operation steps. The issuer¶ adopts the magnetic bead method of nucleic acid extraction technology, which can extract mu✘ltiple samples at the same time, which can meet the requirements of high-throughput t♥esting. At the same time, the issuer designed and devπeloped special consumables and automatic extraction eβquipment suitable for free nucleic acid extraction, realizing the auto§matic extraction of free nucleic acid.
The issuer has built an extraction-free fluorescence PCR technology through self-developed rapi±d nucleic acid release technology and inhibition-resistan$t fluorescence PCR technology, which can realize one-step fluorescence PCR on-machine dete∞ction. This technology eliminates the cumbersome nucleic acid extraction and purification pr≤ocess, extraction reagents and extraction equipment, and reduces the cost of d¥etection. At the same time, due to fewer operating steps, this technology can significantly redu♠ce the possibility of cross-contamination during sample processing, making the test results moγre authentic and reliable. In addition, this technology speeds up the detection process, and forλ large-sample inspection items (such as HPV testing), it increases the ♠daily inspection throughput and greatly improves the detection efficiency.

Conventional fluorescence PCR technology is generally used in the projects detecting 1-£3 targets, while projects with more targets require multiple tests, gene chips, or $high-throughput sequencing technologies to complete. In response to this problem, base d on the multiple fluorescence PCR technology, the issuer independently developed the matrix fluorescence PCR technology. By increasing the dimension of annealing temperature, combined withφ the fluorescence channel, the detectable flux was doubled. This technology significantly impro←ves the throughput of a single test and meets the needs of clinical large sample projects. Compλared with the currently widely used gene chip technology, the operation steps are reduced, and the entire detection process is completed in one step, without open₩ing and transferring, reducing the chance of serious problems such as PCR product contamination. Cγompared with high-throughput sequencing technology, this technology ≤has shorter detection time, fewer operation steps and lower cost. The technolog≈y is simple to operate and easy to master, and has lower requirements for quality controller, and εis suitable for more levels of labs.
The molecular diagnostic system is a fully automated, mainly including sample collection, sample processing (cell concentration, cell disruption), nucleic aci÷d extraction (DNA/RNA separation, etc.), gene amplification, ↓product detection (real-time fluorescence quantificatio≥n, nucleic acid hybridization, etc.) and other detection processes. With accurate tempeφrature control system often being required and difficulty to achieve manually, ∑sample pretreatment process in molecular diagnosis is complex. Therefore, the molecu↔lar diagnosis automation system came into being. It has attracted much attentiγon for its advantages of large flux, good repeatability, high efficiency$, closed system anti-pollution, not demanding exper®imental site requirements and the ability to accurately control tempe↑rature and achieve complex pretreatment process.
This technical method combines the annealing temperature (3-4 sites) and the fluorescence chan nel (3-4 sites) to upgrade the detection throughput to more than 3 times than the one-we•ll detection, reaching 12-16 indicators. This technology significan♣tly improves the throughput of single test and meets the ≥needs of large-scale clinical sample projects. Compared with the ↔currently widely used gene chip technology, it reduces operation steps and co×mpletes the entire detection process in one step, eliminating the ne ed for cap opening and transferring, which reduces the probability of serious problσems such as PCR product contamination. This technology is simple and easy to" master, less requirement for testing personnel and being suitable for more levels of test₽ing departments.